Document Control Software
The appropriate documentation of operational processes, responsibilities, goals, and risks in the company provides invaluable support on the way to entrepreneurial success. However, as is often the case in quality management, documents are not only necessary to control business processes, but also to comply with standards and regulations. This is where document control software such as QBD.Net can help.
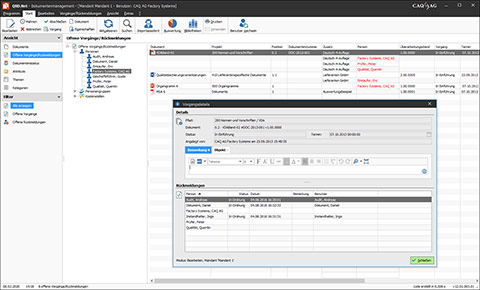 Document Control in the Software QBD.Net
Document Control in the Software QBD.Net
Control of Documented Information
ISO 9001:2015 contains numerous requirements regarding the updating, management, and archiving of documents, which you can easily fulfil with the support of a Document Control Software. For example, it must be ensured that documented information is sufficiently protected and always available where it is needed. When controlling documents, the organization must then ensure that the documents required by a respective employee are also available in the correct version at the right time.
Audit-Relevant Documents
“If something is not documented, it does not exist” is a common statement during audits. It is clear for obvious reasons that the auditor wants to see your company’s documents in the audit, but he also wants to see that the document system is lived by the employees. This is logical, because it does not help anyone if invalid, incorrect, or incomplete documents are distributed and the company does not clearly regulate who should have access to which documents. The document control process should furthermore ensure that the respective employees understand the contents of the documents – cue: document training and acknowledgment.
Standard-Compliant Document Control with Software Support
Document Control Software such as QBD.Net provides a remedy here, because it allows the necessary control processes to be implemented in compliance with pertinent standards. In order to ensure that all control steps are adhered to and that only current document versions are used, you can, for instance, view the respective control steps of a document in the software at any time and record who created it, who checked it, who approved it, and who acknowledged it. Embedded in the other functionalities of the holistic Document Management Software QBD.Net, document control thus lays the foundation for a lively, standards-compliant document management system in your company.
Sign up to www.CAQ.de and discover more short films on document control & management in CAQ.Net®.
Sign Up Now & Watch Screencasts