LIMS Software for Laboratories
Whether acquiring and managing samples in the foodstuffs-industry, planning, developing, and selecting methods and experiments in the pharmaceuticals business or consistently analysing and monitoring pool parameters during galvanization operations: managing the sheer quantity of information and abiding by strict data security regulations are challenges that the research and in-house laboratories of virtually all industries face.
LIMS – Laboratory Information Management System
In order to support laboratories throughout the management of their equipment and information, guidelines such as GLP (Good Laboratory Practice), the FDA’s 21 CFR Part 11 or GAMP (Good Automated Manufacturing Practice) were developed. These generally require adherence to standards such as ISO 9001 and especially ISO 17025 or ISO 15189. Maintaining these complex systems and their associated tools, however, is difficult with pen and paper or spreadsheet solutions alone. This is where CAQ.Net comes into play. It facilitates the targeted implementation of individual software modules in order to create a scalable, integrated, and fully validatable laboratory information management system (LIMS).
Comprehensive Sample Management
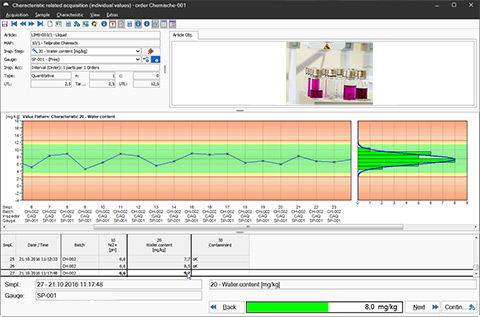 LIMS Software: Compact.Net - Quality Assurance via Control Charts
LIMS Software: Compact.Net - Quality Assurance via Control Charts
The software consistently supports you throughout all analyses, inspections, and examinations in the laboratory and lays the groundwork for comprehensive sample management. Whether preparing, gathering, and distributing samples or performing statistical analyses and creating control charts: the CAQ.Net software provides you with all the tools you require for data acquisition, preparation, and evaluation in the laboratory. In order to ensure data security and the various traceability requirements, the software also contains tools for batch-tracking and analysis specification. The software furthermore supports you throughout the management of deviating analysis results such as Out-of-Specification (OOS) Results, Out-of-Expectation (OOE) Results or Out of Trend (OOT) Results.
The integrated certificate generator allows you to automatically generate certificates directly after an inspection has been completed. For this purpose, the software gathers all the batch information of relevant inspections that is contained in the database and generates a tailor-made certificate accordingly. This certificate may also contain individual raw material certificates such as Certificates of Analysis (CoA). Additional validation inspections after the laboratory analysis, fully automated label printing or the automatic multi-language creation of batch-related certificates conclude the functionalities of the software.
Fully Integrated
CAQ.Net, however, offers a lot more than the basic LIMS-functions required in the laboratory. The system doesn’t just give you a clear overview regarding all laboratory and sample related aspects, but also brings together all important information of the company. It achieves the necessary level of data transparency by bringing together and interconnecting all aspects of your management system. This means that all additional modules for supplier management, audit management, complaint management, document management, task management etc. can be linked and all data used for cross-modular evaluations.
Laboratories that Rely on CAQ.Net