Software for Quality in the Chemical Industry
The chemical industry converts raw materials (oil, natural gas, air, water, metals, and minerals) into polymers and plastics, synthetic rubber, surfactants, dyes and pigments, turpentine, resins, carbon black, explosives, and rubber products or inorganic chemicals like salt, chlorine, caustic soda, soda ash, or acids: all of which are key products that consumers and industries alike rely on. As almost every line of business at some point relies on the products of the chemical industry, the origin and quality of every individual constituent is of paramount importance in this initial phase of the production chain.
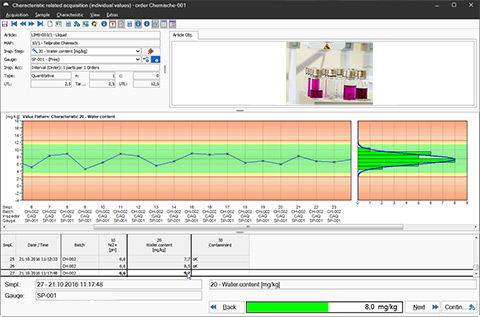 LIMS Software: Quality Assurance via Control Charts
LIMS Software: Quality Assurance via Control Charts
Quality Management Software Solutions
Within its regular operations, the chemical industry must adhere to more rules than merely those of the periodic table. The ICS 71 standards catalogue, for instance, sets the guidelines for chemical technology and production and the American TSCA or regulations by the EPA and further industrial chemical standards outline strict guidelines for the testing and evaluation of the physical and chemical properties of substances. REACH, CLP, and the Certificate of Analysis (COA) are further measures that are applied. As adherence can best be ensured by achieving a consistently high level of quality in products and production processes, our CAQ.Net software solutions provide everything you need in order to be able to manage, improve, and comprehensively document your product quality.
Chemical Companies that Rely on CAQ.Net